Gas bubbles can form when a chemical solution is boiled or warmed. Click card to see definition.

Preclinical Exam Chpt21 Techniques Of Instrument Processing And Sterilization Flashcards Quizlet
Where is the PCD placed in a steam sterilizer.

. A drop of indicator solution is added to the titration at the beginning. The pack duplicates the most complex package to be sterilized indicator is placed in the most inaccessible portion of the pack and the pack in most difficult to sterilize position in the chamber. Chemical indicators can help assess physical conditions and identify procedural errors.
An acidbase indicator eg phenolphthalein changes color depending on the pH. Since some chemical products are poisonous detecting a chemical reaction via smell or taste change is not recommended. Fermentation of Carbohydrates Yellow with gas.
Classify each substance as acidic pH 7 alkaline ph 7 or neutral pH 7. A new base has been produced. -The pen used to check for counterfeit money is a starch indicator.
Click card to see definition. Process integrators internal- Strips placed in packages that change color when exposed to a combination of heat and temperature. They may be used as internal chemical indicators.
Always between a pH of 6 and 8. Redox indicators are also used. At pH 7.
Health indicators provide comparable and actionable information across different geographic organizational or administrative boundaries andor can track progress over time. At the midpoint of a titration. Tap again to see term.
Type 4 - Multi-variable indicators are designed to react to two or more of the variables of the sterilization process that they are designed to monitor. The change in color most likely indicates that a chemical change has occured. Most Type 4 indicators show an acceptable endpoint by.
Chemical indicators are organic substances that are used to determine the endpoint. -All you need an equilibrium reaction with different colored products and reactant. Biological indicators are processed in a Process Challenge Device or PCD.
Why do indicators change colors. -Because of Le Châteliers principle. Such dyes may be used to indicate when the acid base in a solution has been.
Use the Gizmo to find the pH of each of the available substances. A solution of indicator molecules takes on the color of the most abundant form - wither the protonated or the unprotonated form. Tap card to see definition.
Orange coral pink blue yellow. The endpoint has been reached when the color changes. Compare the paper to the pH color chart.
Most of the pH indicators work at a pH range from 3 to 11. There are four different indicators that could be used for this pH range. Exactly when pH pK_a of the indicator.
Process indicators external- Tapes strips or tabs with heat-sterilizative chemicals that change color when exposed to a certain temperature. External indicators that change color when a specific parameter is reached should be applied on the outside of the sterilization packages. Cyanidin red litmus phenolphthalein and phenol red indicator.
What is the color of this indicator in a solution whose pH is 400. Once sufficient silver is added to precipitate chloride as silver chloride excess silver is adsorbed onto the surface. A ph meter determines.
Is the chamber full or empty. Unionized forms essentially have a different color than ionized forms. Click to see full answer.
A physical change has occured. Fluorescein is a type of adsorption indicator. A health indicator is a measure designed to summarize information about a given priority topic in population health or health system performance.
This problem has been solved. Five indicators of a chemical change are color change temperature change precipitate formation gas bubble formation and smell or taste change. The endpoint of a titration is the point where the indicator just changes colour.
Anions undergo reduction because they gain electrons. The dye is used to detect the completed reaction of the silver ion with chloride. Which type of ion usually undergoes reduction.
Oxidation is a loss of electrons. If the indicator is placed in a buffer solution of Ph 456 what percent of the indicator will be present in the acid. Indicators change color when an acid and a base are mixed together.
Fluorescein combines with adsorbed silver to produce a color change from greenish-yellow to red. Fermentation of Carbohydrates Red when basic Yellow when acidic What are the positive results. Generally over a range of 1 or 2 pH units.
These are recommended to be included in each load. Indicators change their colors at a certain pH range due to ionization. The ionization constant Ka of an indicator HIn is 10x10-6.
What is the color of the pH paper. For a strong acid and a strong base such as NaOH and HCl the final solution is neutral at pH 7. Tap card to see definition.
The equivalence point is when the ratio of the reactants is in the amounts specified by the equation. A substance that changes color in response to a chemical change. What is the pH of ammonia.
What is the color of the pH indicator. The chemical present in the indicator ink reacts to one or more of the critical parameters of the sterilization process and undergoes a chemical reaction to alter and change the color of the indicator ink to its endpoint color. The color of the nonionized form is red and that of the ionized form is yellow.
A new acid has been produced. PH indicators change color at their pKa where the identity of the most abundant form changes. Both external and internal indicators can be used.
Indicators change color in acids or bases. Indicators are generally weak acids or weak bases. Acid-base indicators change color_____ a.
The second type of chemical indicator utilizes one or more chemical reactions to bring about a chemical change. See the answer Show transcribed image text Expert Answer. Many soluble dyes change their color with changes in pH a measure of the concentration of acid in a solution.
Ideally you would want these points to coincide. What happens during oxidation. The pH of a solution by measuring the voltage between the two electrodes that are placed in the solution.
Click again to see term.

Preclinical Exam Chpt21 Techniques Of Instrument Processing And Sterilization Flashcards Quizlet
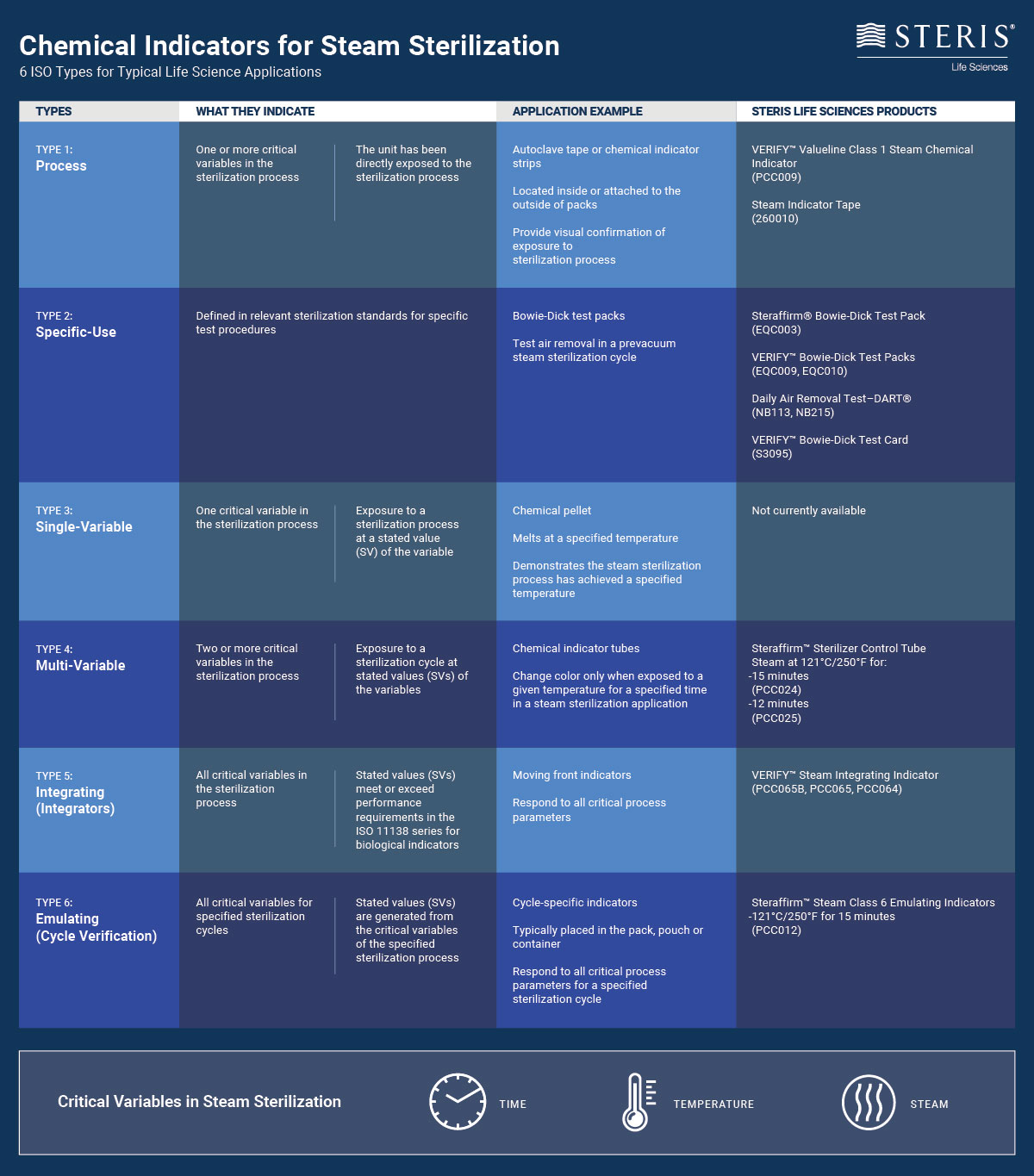
6 Iso Types Of Chemical Indicators For Steam Sterilization Steris

Exam 1 Qmb 3602 Flashcards Quizlet

Personality Types Powerpoint Charts Powerpoint Charts Powerpoint Chart Templates Powerpoint
0 Comments